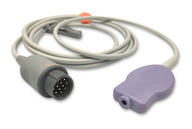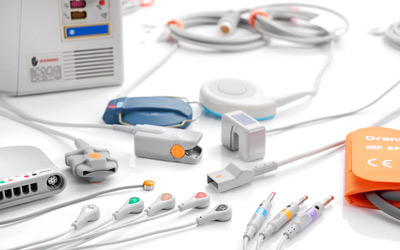
OEM Medical Cable Manufacturing for Patient Monitoring Systems
Engineered for Precision. Built for Reliability. Backed by Certification.
Orantech is a trusted OEM manufacturer of original medical-grade cables and components for patient monitoring systems. Our products are engineered for precision, durability, and signal integrity — built from the ground up to meet the specifications of medical device manufacturers worldwide. From SpO₂ and ECG to IBP and fetal monitoring, every cable is produced in our ISO 13485:2016-certified, FDA-registered, and CE-marked facility in Shenzhen.
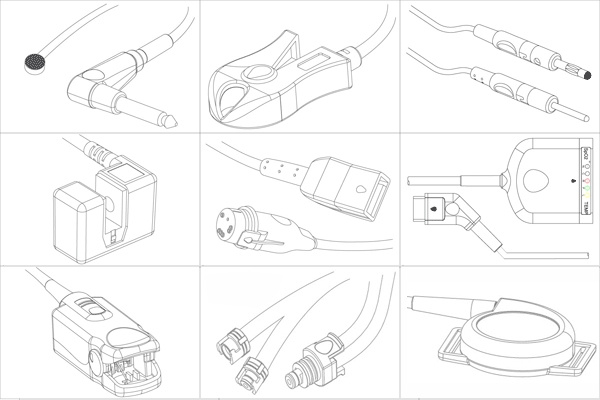

Orantech offers comprehensive OEM services, providing complete control over design and product modifications. Our full-service fabrication facilities can manufacture custom components, assemblies, or complete systems from your drawings or 3D models.
// Read more about our servicesWhat’s New at Orantech
Stay informed about compliance updates, and manufacturing innovations.
What to Look for in a Medical Cable Manufacturer
Choosing the right medical cable manufacturer is critical for ensuring patient safety, data integrity, and regulatory compliance. Orantech stands out with ISO 13485 certification, FDA registration, and 510(k) support—backed by custom OEM services, seamless compatibility with Philips, GE, and Masimo systems, and transparent quality testing. From design to delivery, we engineer every cable for performance and long-term reliability.
Explore More News →OEM Medical Cable Assemblies
Discover our ISO 13485-certified custom cable manufacturing for patient monitoring and diagnostic systems — built for durability, safety, and seamless OEM integration.
// View OEM AssembliesPatient Monitor Cables
Premium reusable cables compatible with GE, Philips, Masimo, and more — engineered for signal integrity, clinical reliability, and backed by a 12-month warranty.
// View Patient Monitor CablesFetal Monitoring Cables
High-quality fetal cables and accessories designed for use with GE systems and OEM integrations — durable, accurate, and fully reusable.
// View Fetal Monitoring SolutionsOrantech, a leading manufacturer of cables and cables, provides top-quality products from our Shenzhen, China facilities. Equipped with industry-leading equipment for research, development, and production, we are TUV ISO 13485:2016 certified, FDA registered, and hold 510K clearance and CE certificates.
// Explore more